Breakthrough in Male Contraception
Currently, the majority of available birth control options are designed for women. However, biotech company Contraline has announced that its non-hormonal and reversible male contraceptive, ADAM, has reached a significant milestone in its clinical trials.
In a recent news release, Contraline revealed that ADAM has demonstrated effectiveness and safety 24 months into its first human clinical trial. Although the clinical results have not yet been published in a scientific journal, the company plans to release additional data during the American Urological Association (AUA) meeting on April 26.
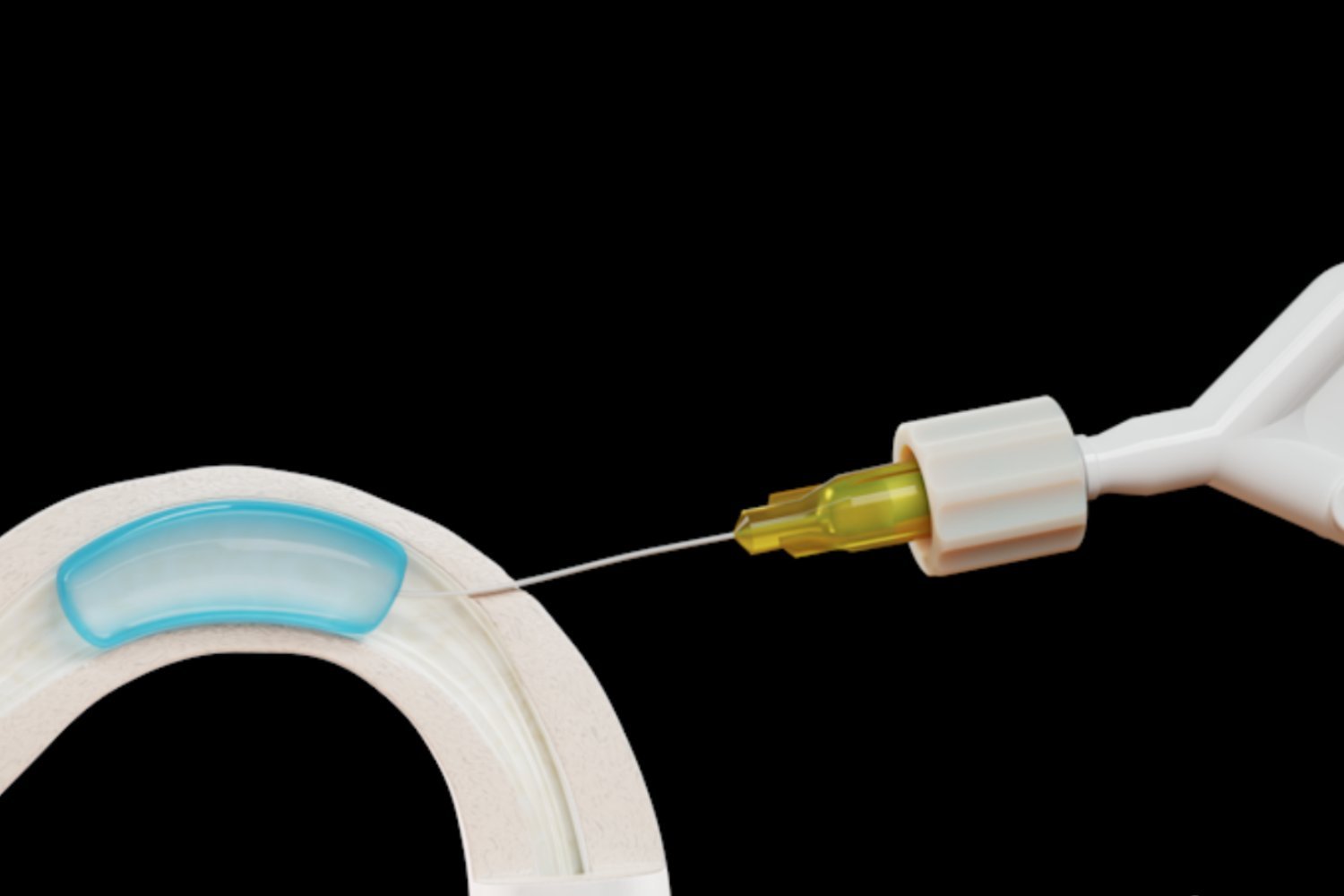
ADAM is a water-soluble hydrogel that is injected into the vasa deferentia, the two tubes that carry sperm from the testicles to the urethra, through a minor procedure. The implant blocks sperm while allowing ejaculation, and Contraline has been marketing it as a potential long-term, reversible alternative to condoms and vasectomies.
According to Alexander Pastuszak, Contraline’s Chief Medical Officer, “Our goal was to create a male contraceptive option that lasts two years, directly addressing consumer needs. These findings confirm that ADAM can achieve the intended lifespan. We remain optimistic about its safety, efficacy, and reversibility, and its potential to provide men and couples with greater reproductive control.”
The milestone is based on two participants in the first human clinical trial showing azoospermia, or no sperm in their ejaculate, at 24 months. In a previous news release, Contraline reported that ADAM caused a 99.8% to 100.0% reduction in the number of moving sperm within 30 days of implantation.
These results “bring us one step closer to transforming the contraceptive landscape,” said Kevin Eisenfrats, co-founder and CEO of Contraline. They suggest “that it is possible to achieve similar levels of efficacy as long-acting female contraceptives like IUDs. Ultimately, I’d like to make ADAM a ‘no-brainer’ for men when it comes to considering their options for contraception.” IUDs, or intrauterine devices, are small female contraceptive devices inserted into the uterus.
As stated in the recent news release, none of the participants have reported serious adverse events or faced unexpected safety concerns. Researchers conducting the clinical trial will continue monitoring other participants at the 12-, 15-, 18-, and 21-month marks through lab and at-home sperm testing. Furthermore, Contraline has received full regulatory approval to start the study’s second phase.
However, Jon Oatley, a professor at Washington State University’s School of Molecular Biosciences, noted that there is currently no public data confirming the reversibility of the ADAM implant, and researchers still don’t know the long-term effects of blocking the vasa deferentia, as he told The Guardian. Oatley also suggested that most men would prefer a contraceptive pill or patch over a surgical procedure.
Despite this, data from 2017 to 2019 shows that 10.4% of women between the ages of 15 and 49 used long-acting reversible contraceptives, such as IUDs or other contraceptive implants requiring procedures. This is less than four points behind the pill’s 14%. If ADAM proves to be safe and effective, it’s possible that a significant number of men will choose the long-term efficacy of an injection over other contraceptives.
Source Link